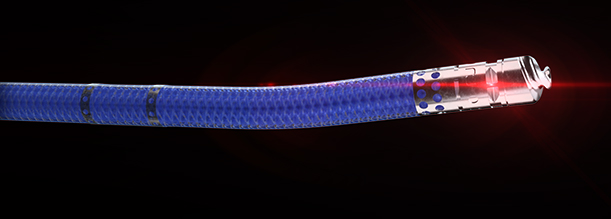
VISUALIZATION.
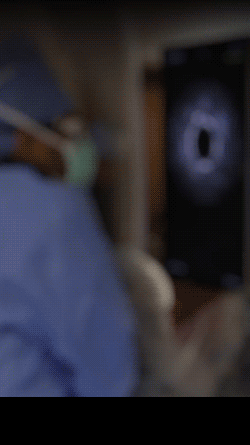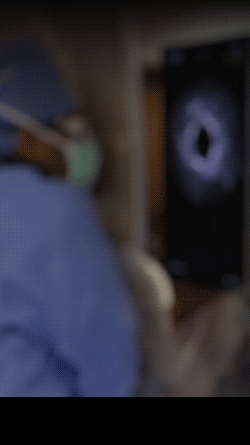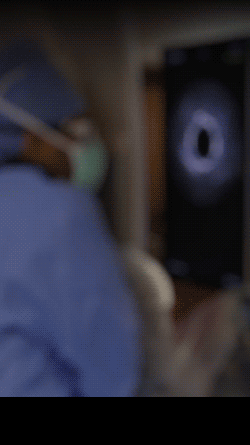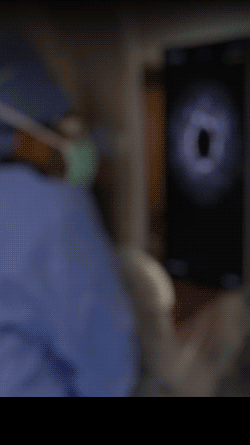
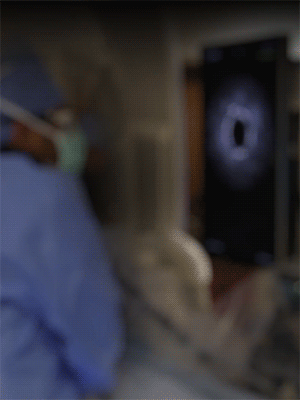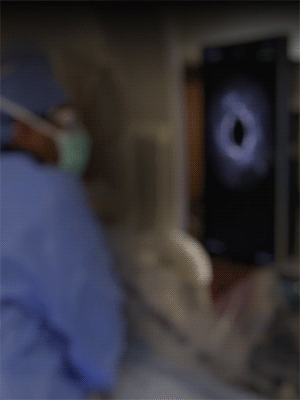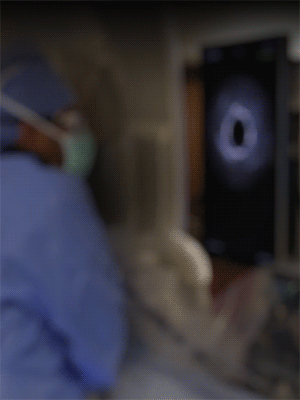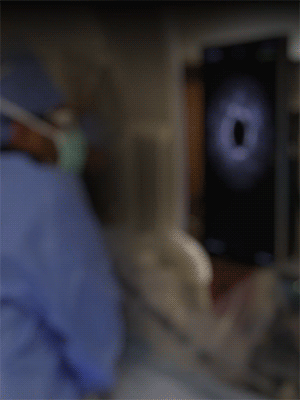
Knowledge is power. The ability to see inside the artery during treatment gives physicians a lot more knowledge of the clinical problem and the power to do something about it in real time.

-
ADVENTITIA
The supportive and outermost layer of the artery is also the most sensitive to injury during treatment.
-
External Elastic Lamina (EEL)
Science has proven that when this thin bright border is breached, the body may generate an aggressive healing response that leads to renarrowing of the artery.
-
Media
The middle or muscular layer of the artery shows up as a dark band with OCT imaging.
-
DISEASE
The deposits that cause the narrowing in the artery can easily be characterized and differentiated from the arteries natural structures because it’s non layered and amorphous.
PROVEN PRECISION.
The Lumivascular technology has generated some of the most compelling data
in the industry to date - proving that being able to see and treat at the same
time can provide best in class clinical outcomes.
vision ide 6 month
lumivascular ATHERECTOMY
130 PATIENTS | 20 SITES | 164 LESIONS | PRIMARY COHORT
LUMINAL GAIN | 72%
-
PRE-STENOSIS
-
PANTHERIS STANDALONE
-
POST-PANTHERIS +/- ADJUNCTIVE THERAPY
adventitia analysis
EFFICACY
Safety
follow-up data
lumivascular ATHERECTOMY
freedom from tlr
SOURCE: VISION (FULL COHORT, BY LESION)
-
12 MONTH (N=89)
-
18 MONTH (N=79)
-
24 MONTH (N=72)
FREEDOM FROM TLR & PRIMARY PATENCY*
SOURCE: EUROPEAN CASE SERIES (GRAZ, AUSTRIA) BY LESION
-
FREEDOM FROM TLR (12 MONTH) (N=33)
-
PRIMARY PATENCY* (12 MONTH) (N=33)
* Patency is defined as PSVR < 2.4
CONNECT II DATA
LUMIVASCULAR CTO CROSSING
100 PATIENTS | 14 SITES | 100 LESIONS
LUMINAL CROSSING
-
93% = TRUE LUMEN
-
7% - SUBINTIMAL

SAFETY.
Lumivascular is a radiation free technology and using it may reduce procedural radiation exposure for doctors.
Learn More about how lumivascular is limiting radiation exposure